The Sanner Group, Gilero’s parent company, brings 130+ years of experience and a global
manufacturing network, being a global leader in the development and manufacturing of
medical devices, as well as pharmaceutical and nutraceutical packaging solutions. With
state-of-the-art manufacturing facilities in the United States, Asia, and Europe, Sanner offers
end-to-end services, spanning engineering development, clinical trials, and large-scale
manufacturing.
Our Expertise
Global Device Manufacturing
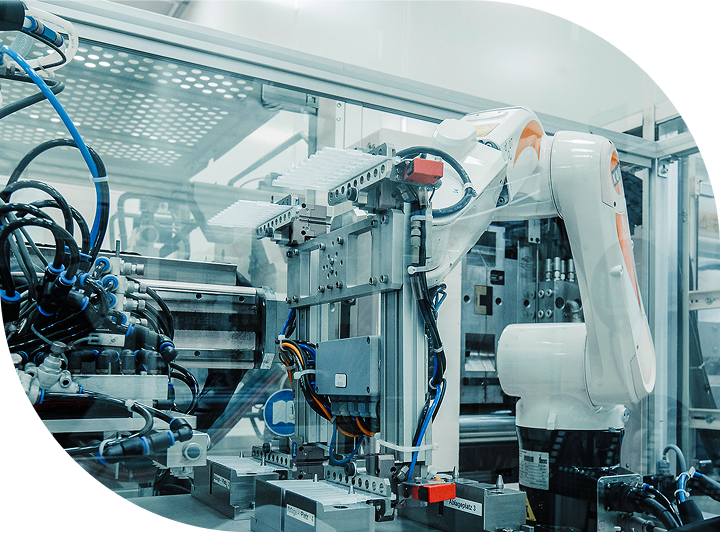
Scalable solutions for global
device manufacturing.

Small and Large-Scale Production
Sanner’s global manufacturing network spans 300,000 square feet and is built to support both small-batch and high-volume production with the flexibility and quality demanded by the medical and healthcare industries.

Our facilities are strategically located in Europe, Asia, and the United States.

Our operations are ISO 13485, ISO 9001, ISO 15378 certified for fully GMP (FDA) and MDR-compliant manufacturing.

In total, we have 11,000 sqm of modular cleanroom space (ISO Class 7 & 8), with dedicated expansion zones for future equipment and cleanroom installations.
Packaging and Moisture Management
With over 60 years of expertise in physical and chemical stability packaging for moisture-sensitive pharmaceutical products, we provide high-quality desiccant primary packaging and drop-in solutions, including fully customized options to meet specific needs.

Drop-In moisture and odor management solutions are tailored to meet the custom specific shelf-life requirements of moisture sensitive products.

The desiccant built-in solutions are a cost-efficient alternative to desiccate differently. Sanner offers a variety of closer/container systems including the patented TabTec® CR.

The proprietary Atmo Guard System® simulates internal humidity using product-specific chemical and physical data, streamlining development and improving long-term product stability.


Seamless Manufacturing Transfer
We integrate design transfer and industrialization into a unified, structured process that ensures continuous alignment from early development through full-scale production. This approach links product design and manufacturing in a way that supports regulatory compliance, production readiness, and long-term scalability.

Our global manufacturing team is highly experienced in optimizing injection molding tooling, ensuring precision and efficiency in production.

Using digital twins and mold flow simulations, we optimize mold designs for manufacturability, identifying and addressing potential issues early in the process to reduce risk and rework.

Talk to an Expert
Ready to take the next step in product development? Connect with the Gilero team to explore tailored solutions—from concept through commercialization.




